Teva's pursuit of J&J boosted by FDA acceptance of filing for approval of long-acting schizophrenia drug | FiercePharma
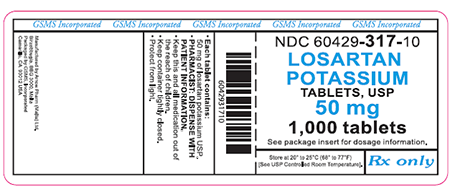
Teva Pharmaceuticals USA, Inc. Expands Voluntary Nationwide Recall of Losartan Potassium to 50 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply, Inc. | FDA

Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of all Amlodipine/Valsartan Combination Tablets and Amlodipine/Valsartan/Hydrochlorothiazide Combination Tablets that are Within Expiry | FDA

Teva and MedinCell Announce FDA Acceptance of New Drug Application for TV-46000/mdc-IRM as a Treatment for Patients with Schizophrenia | Business Wire