Emboldened By Sage's Zulresso Slip, Marinus Is Set To Succeed In PPD And Orphan Epilepsy (NASDAQ:MRNS) | Seeking Alpha

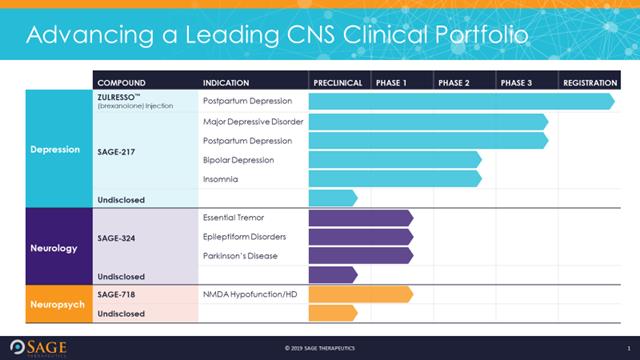
Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration | Sage Therapeutics, Inc.

Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration-CliniExpert
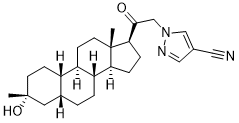
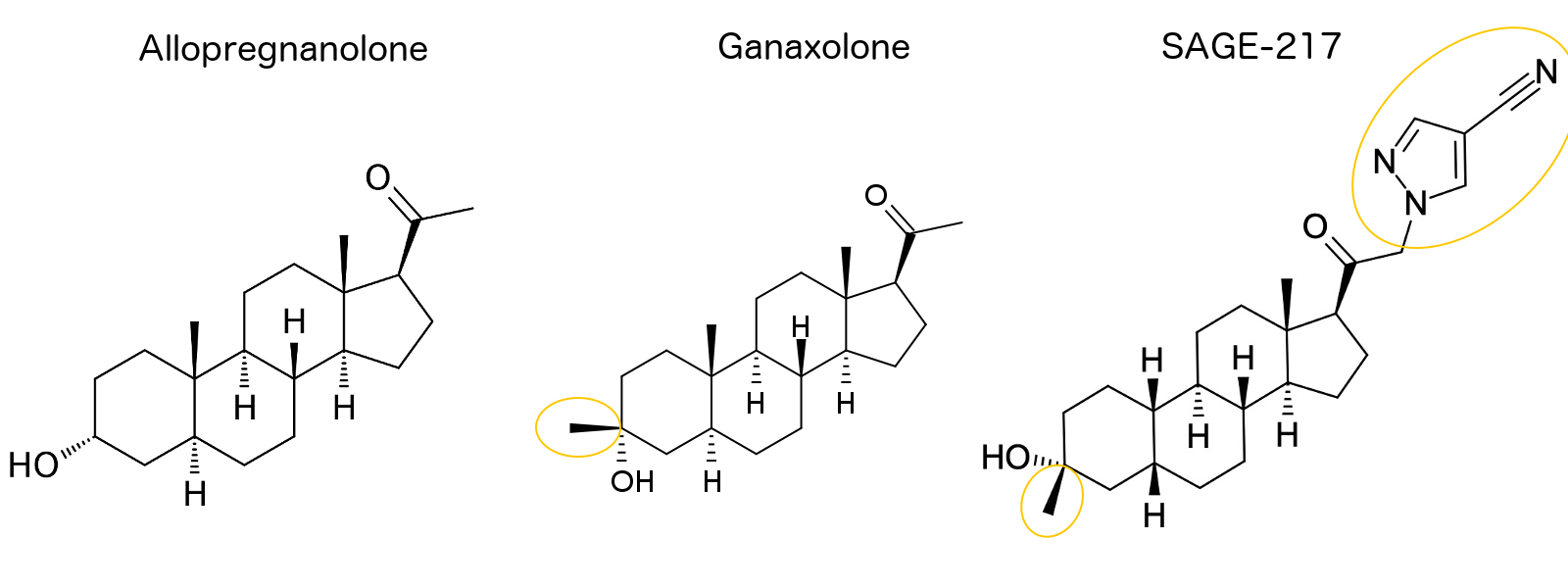
Sage Therapeutics Receives FDA Breakthrough Therapy Designation for SAGE-217 for the Treatment of Major Depressive Disorder - Chemdiv

Sage Therapeutics and Biogen Report Positive Phase 3 Data Showing Zuranolone Significantly Reduced Depressive Symptoms After Two Weeks

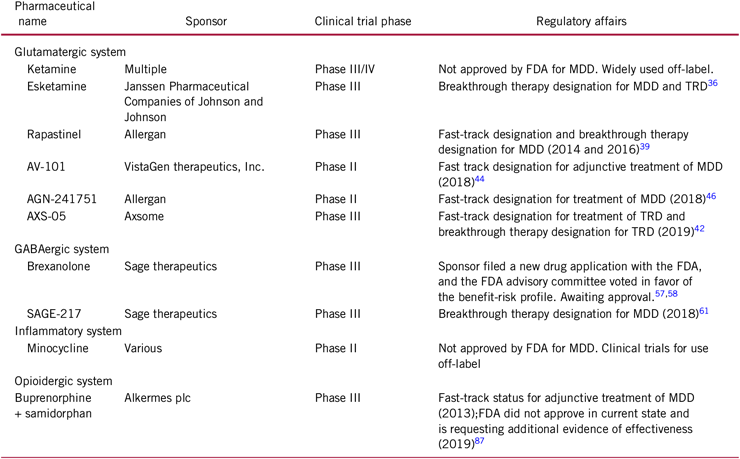
Sage Therapeutics Reports Topline Results from Pivotal Phase 3 MOUNTAIN Study of SAGE-217 in Major Depressive Disorder
Sage Therapeutics Forges $575 Million Deal With Shionogi to Market Depression Drug in Parts of Asia | BioSpace

Sage Therapeutics and Biogen Announce Positive Pivotal Phase 3 Results for Zuranolone, an Investigational Two-Week, Once-Daily Therapeutic Being Evaluated for Major Depressive Disorder | Business Wire

SAGE Therapeutics shares rocket after FDA clearance to expedite its depression post-partum drug SAGE-217

Sage Therapeutics and Biogen Announce New Analyses from the LANDSCAPE Clinical Development Program of Zuranolone in MDD Presented at the American College of Neuropsychopharmacology (ACNP) Congress | Business Wire
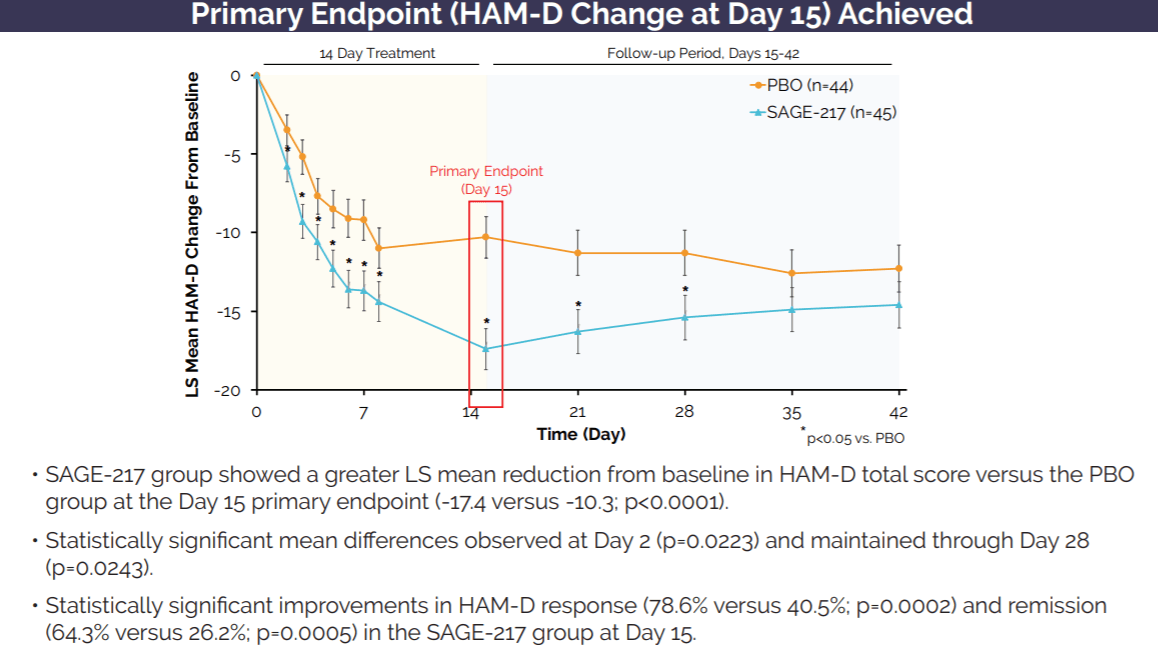
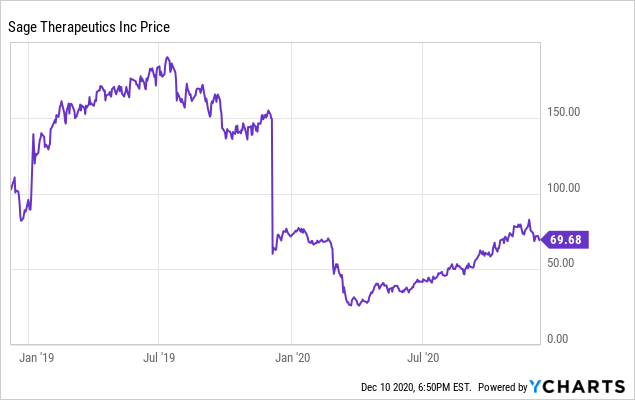
Sage Therapeutics: Zuranolone's Multi-Billion Dollar Market Opportunity More Than Justifies Company Valuation (NASDAQ:SAGE) | Seeking Alpha

Sage Therapeutics Reports Positive Top-Line Results from Phase II Placebo-Controlled Trial of SAGE-217 in MDD - Drug Discovery and Development