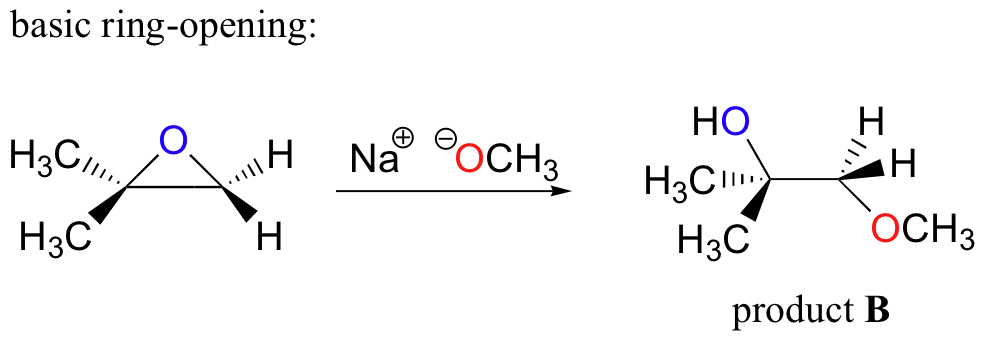
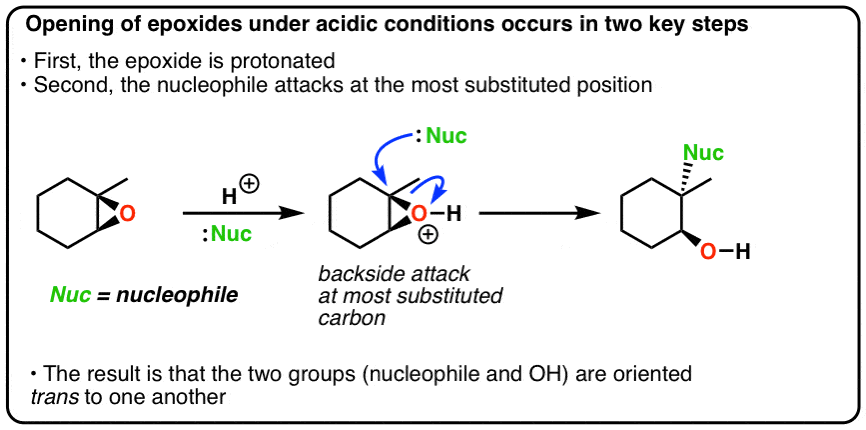
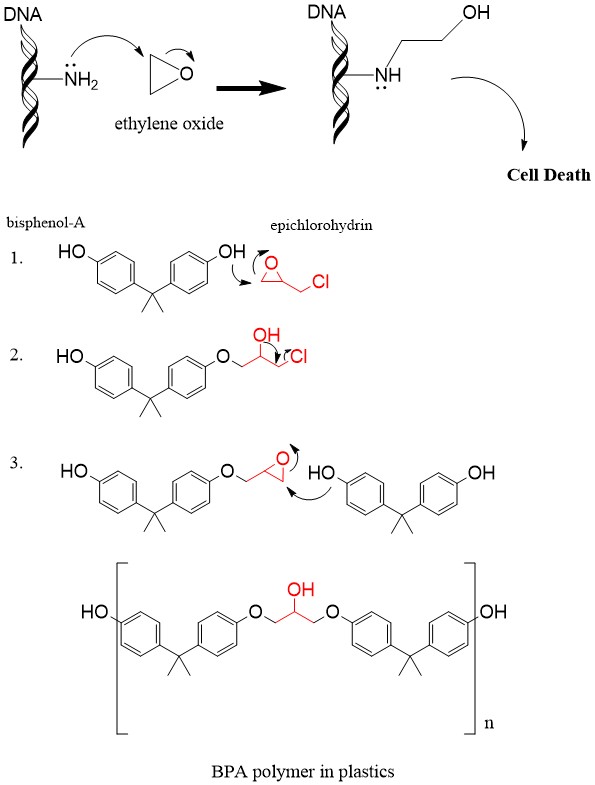
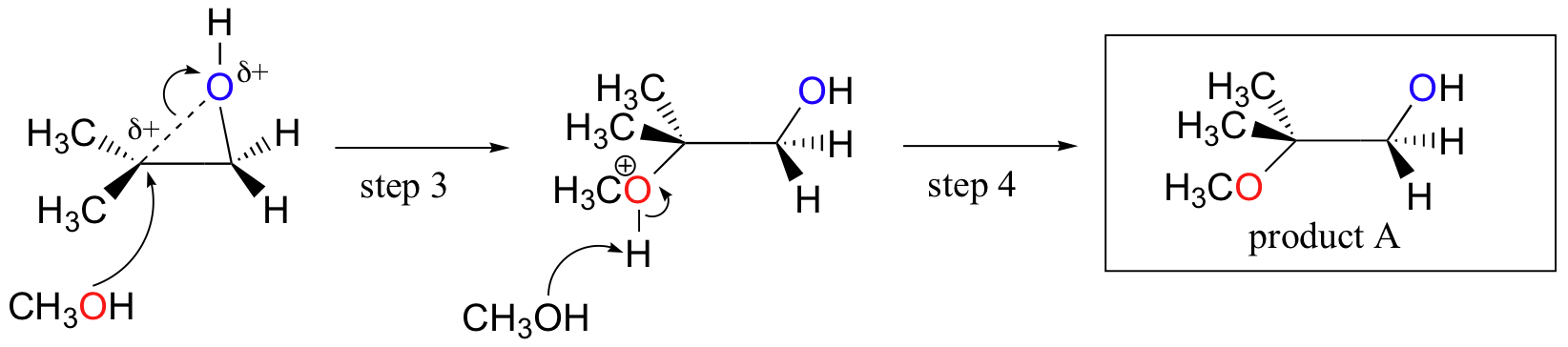
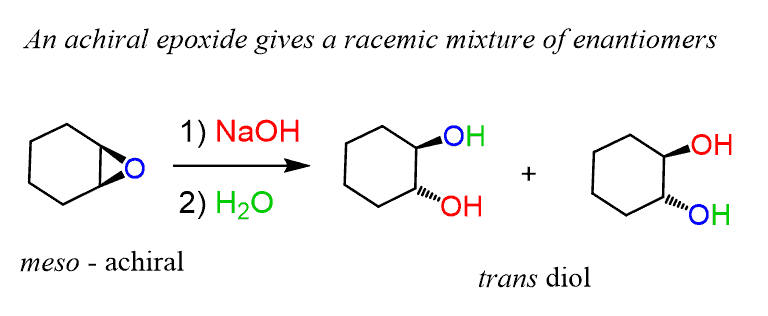
organic chemistry - Regioselectivity of acid-catalyzed ring-opening of epoxides - Chemistry Stack Exchange

Unexpected Highly Efficient Ring-Opening of Aziridines or Epoxides with Iodine Promoted by Thiophenol

Why does ring opening reaction (of lactones) often follow nucleophilic pathway and not electrophilic?

Carbon Tetrabromide: An Efficient Catalyst for Regioselective Ring Opening of Epoxides with Alcohols and Water
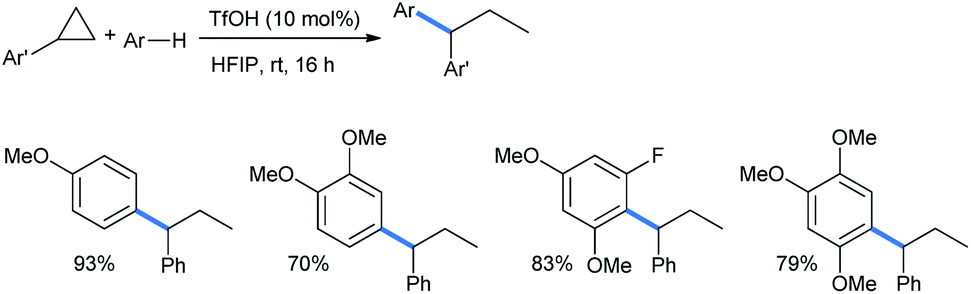
Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes - RSC Advances (RSC Publishing) DOI:10.1039/D0RA05111J

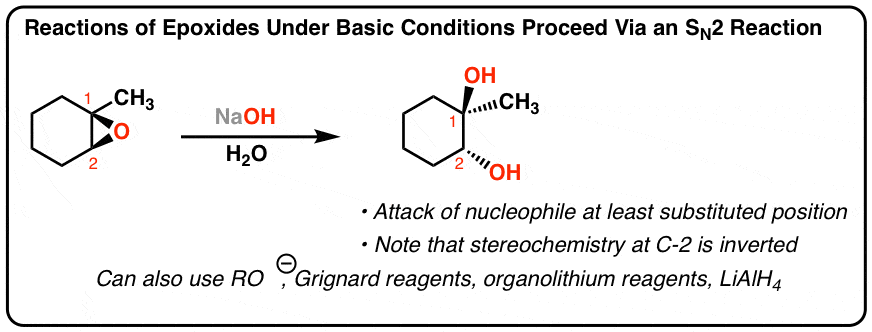
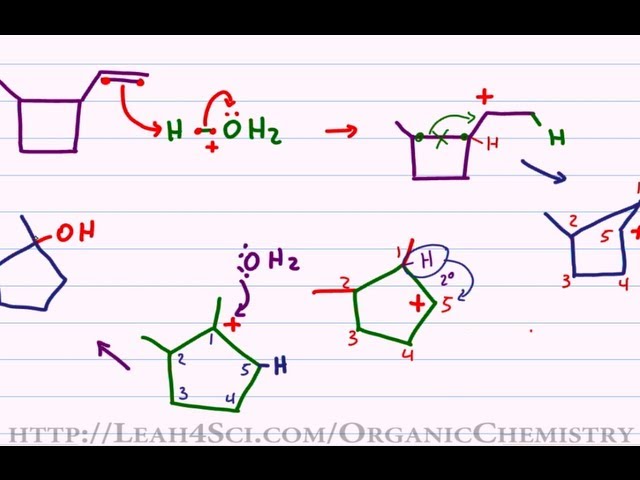

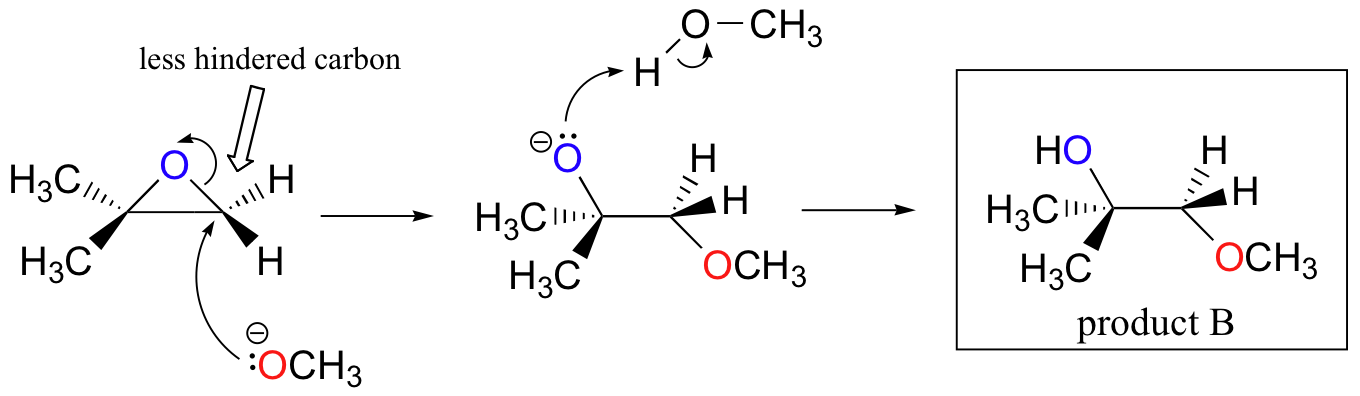


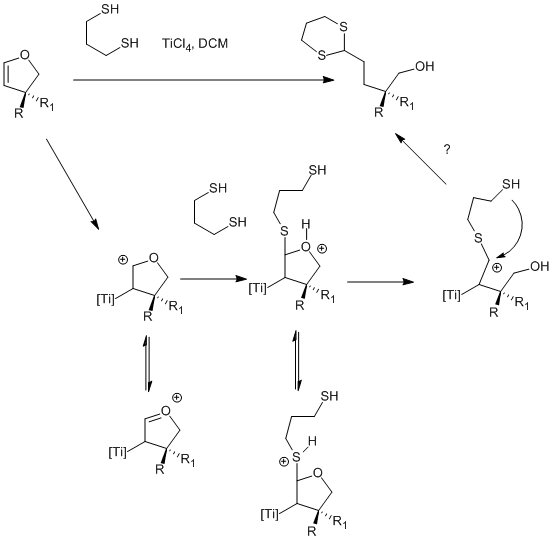

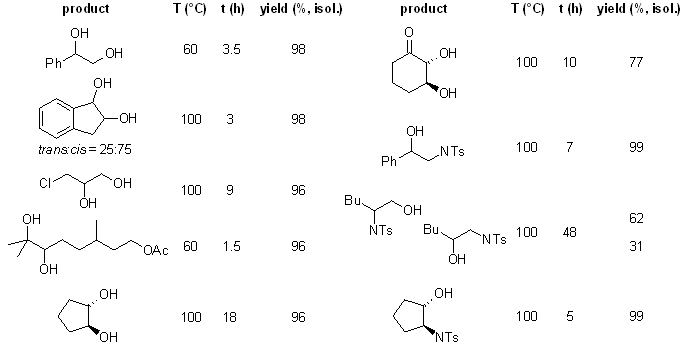
.gif)