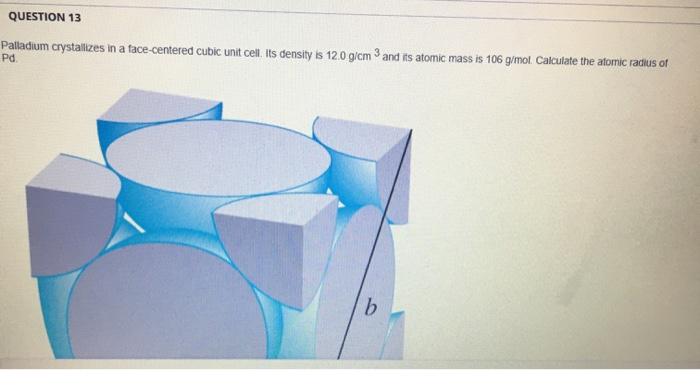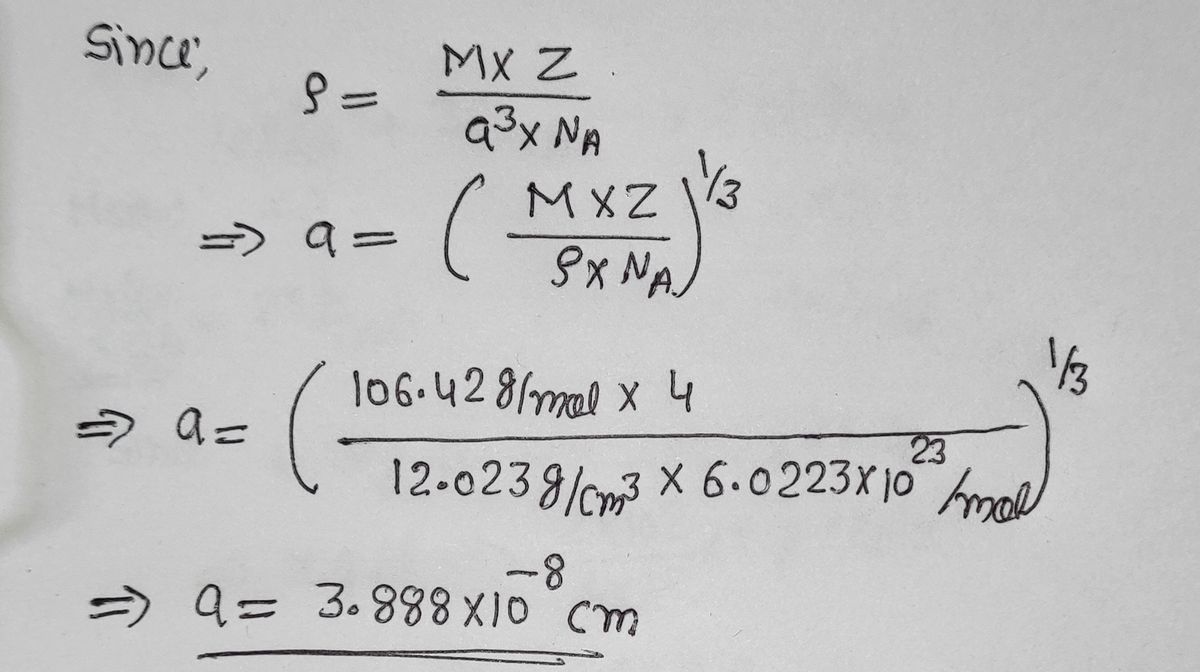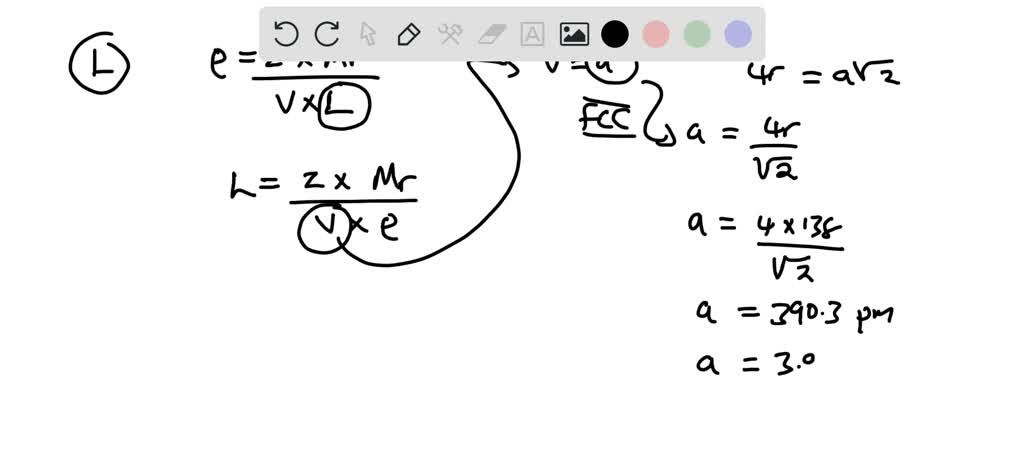
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of $12.0 mathrm{~g} / mathrm{cm}^{3}$, a radius of $138 mathrm{pm}$, and a molar mass of $106.42 mathrm{~g} / mathrm{mol}$. Use this

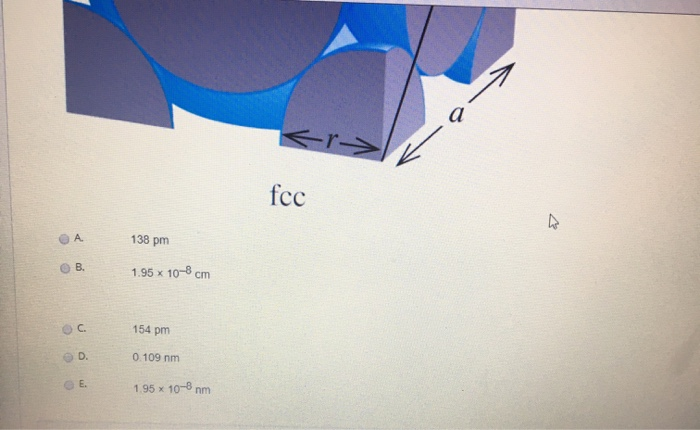
SOLVED:16. Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 glem'. What is the atomic radius of Pd? A. 138 pm B. 1.95 * 10 8 nm C.1.95 * 10-8 cm D. 154 pm E. 0.109 nm

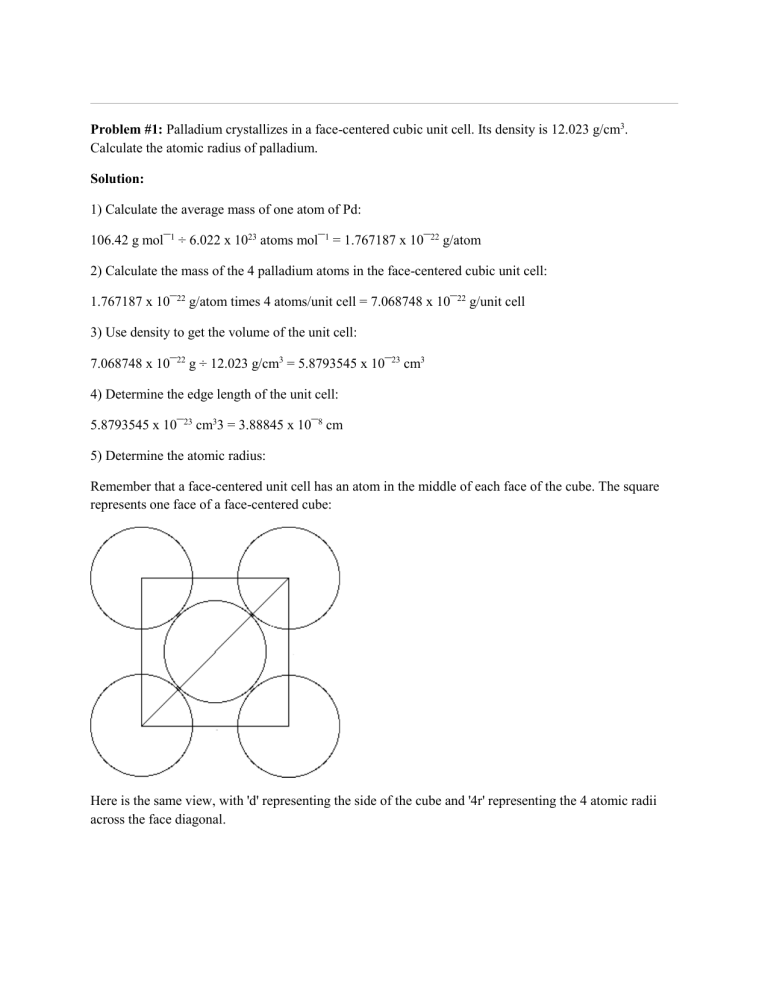
Problem #1: Palladium crystallizes in a face-centered cubic unit cell Its density is 12023 g/cm 3 Calculate the atomic radius of palladium | Course Hero

Document - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero
Problem #1: Palladium crystallizes in a face-centered cubic unit cell Its density is 12023 g/cm 3 Calculate the atomic radius of palladium | Course Hero
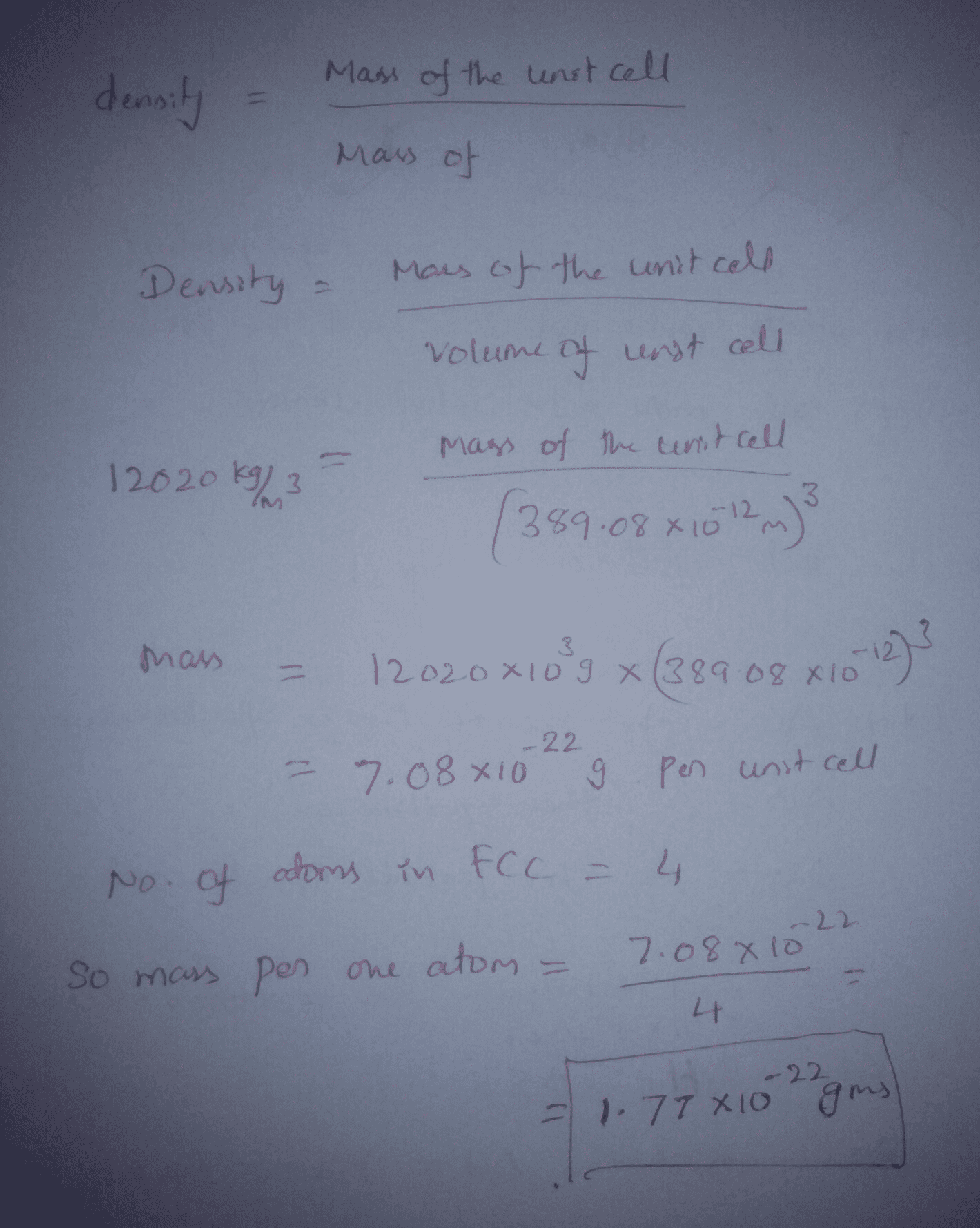
OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...
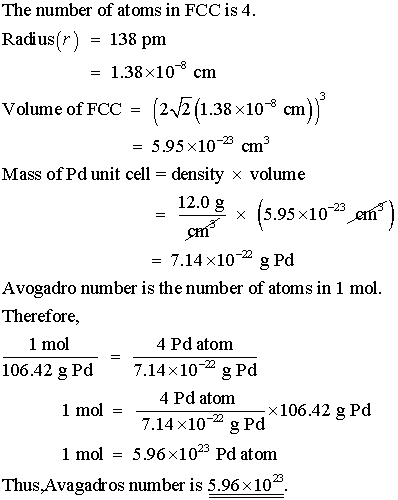
OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...

Copper crystallizes in a cubic structure. If the density of the metal is 8.% g/cm^3 and the length of the unit cell edge is 361 picometers, find the number of atoms in
If the radius of palladium is 248 pm and the lattice type is body centered cubic, what is the - Sarthaks eConnect | Largest Online Education Community

Problem.docx - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero