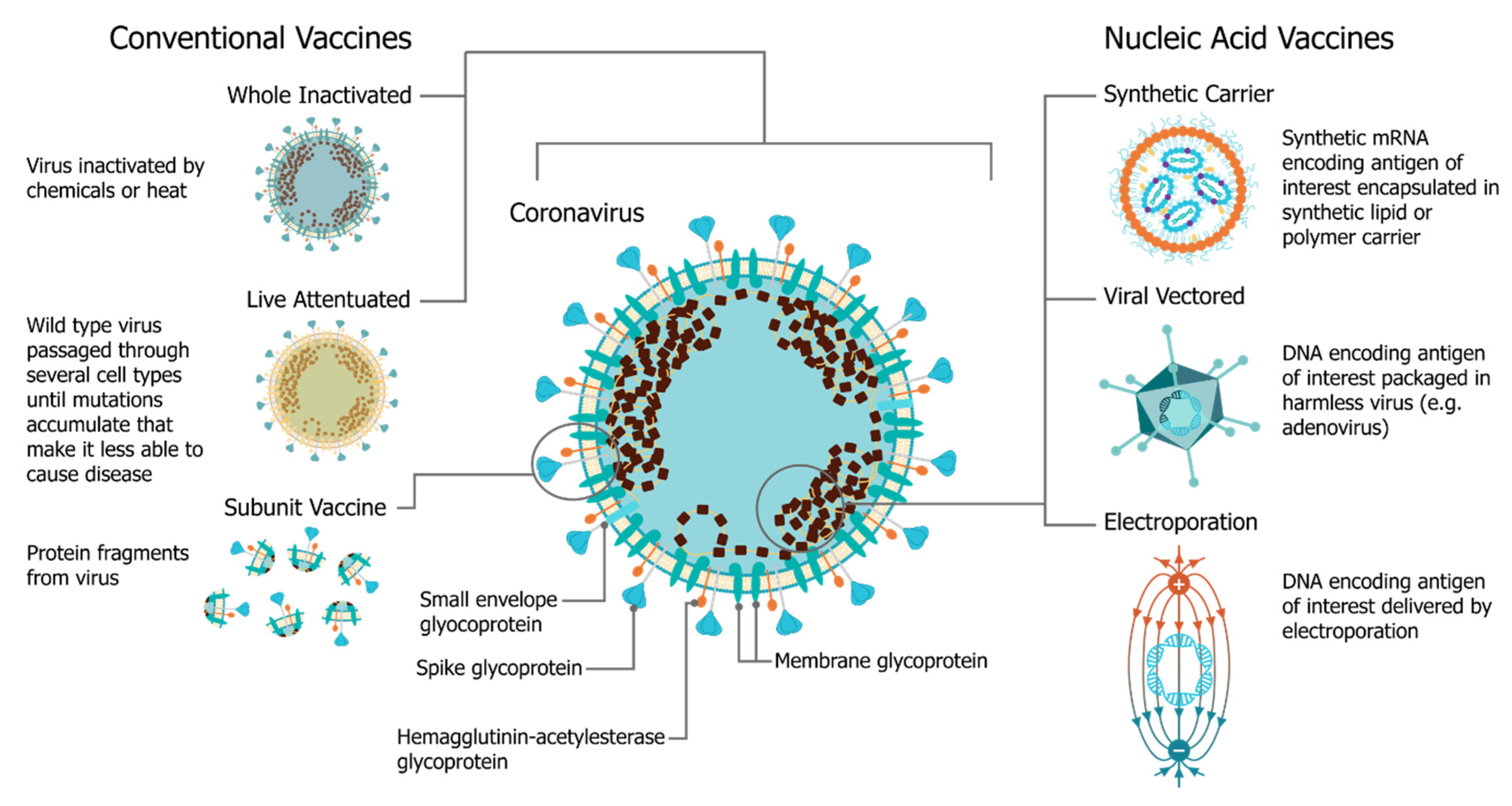
Frontiers | Current Efforts in the Development of Vaccines for the Prevention of Zika and Chikungunya Virus Infections | Immunology

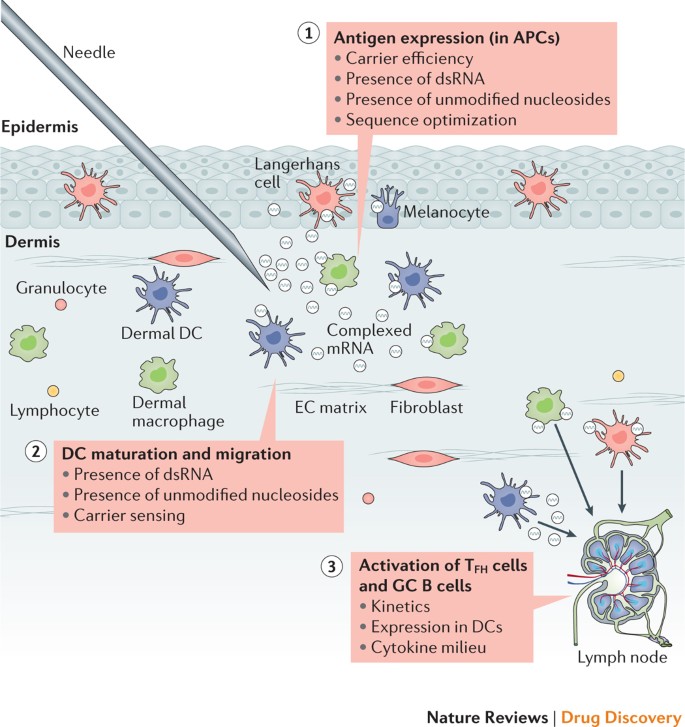
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC
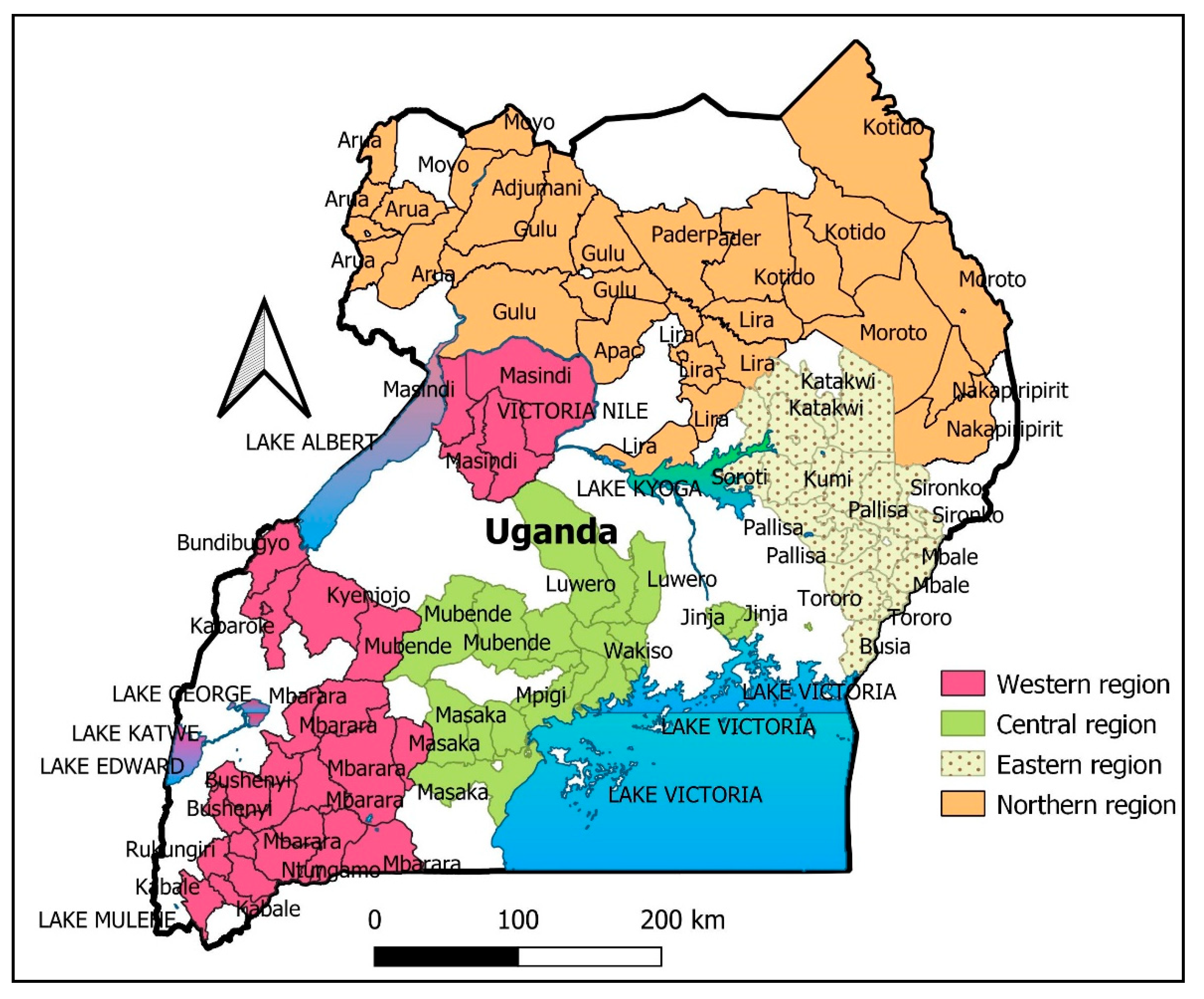
Vaccines | Free Full-Text | A Descriptive-Multivariate Analysis of Community Knowledge, Confidence, and Trust in COVID-19 Clinical Trials among Healthcare Workers in Uganda | HTML

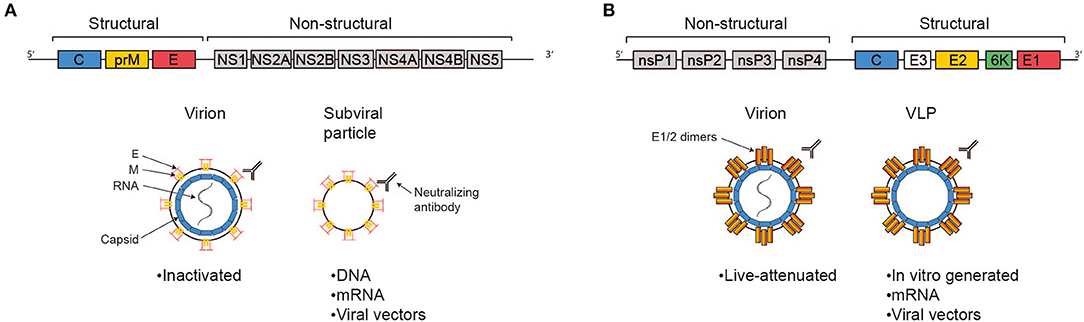
Biomaterials and nanomaterials for sustained release vaccine delivery - Luzuriaga - - WIREs Nanomedicine and Nanobiotechnology - Wiley Online Library
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-C

A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC

A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC
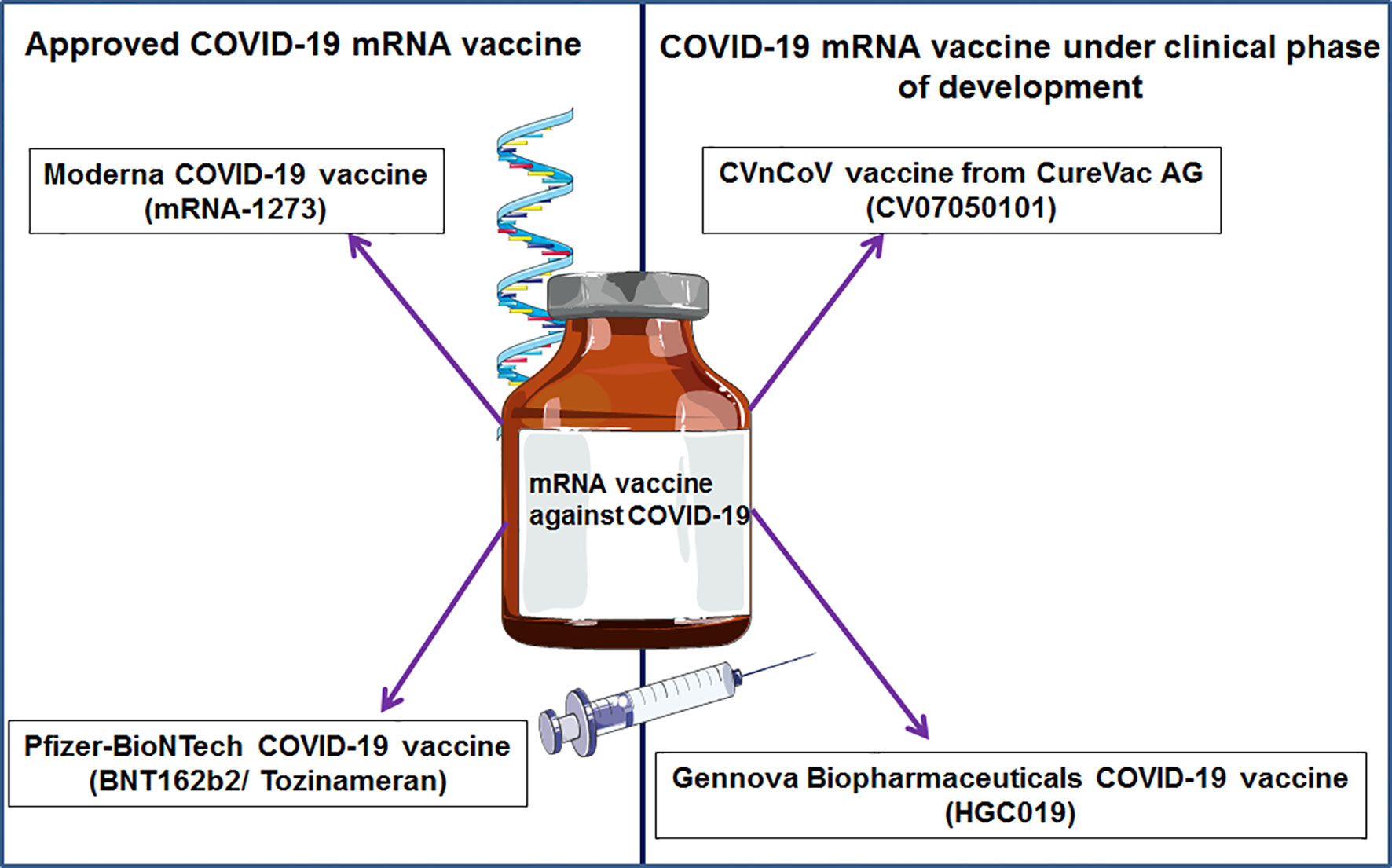
Frontiers | From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape | Immunology
Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses

Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination | Science Immunology