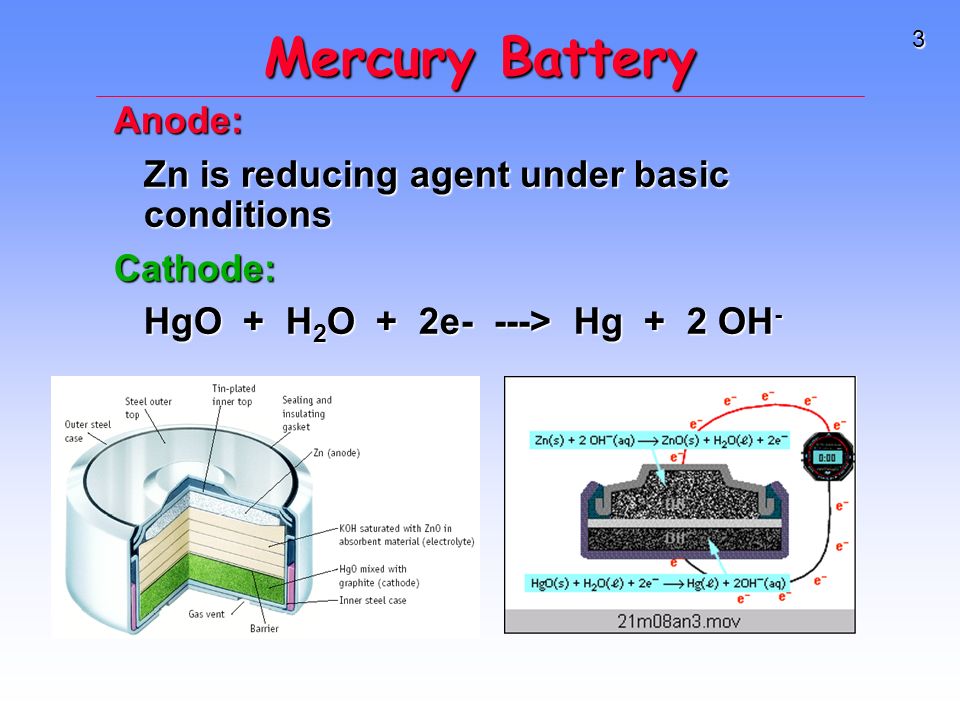
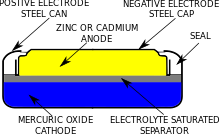
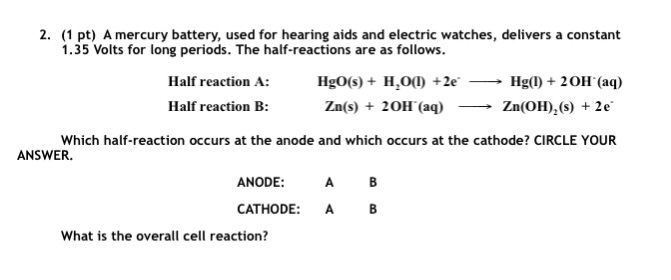
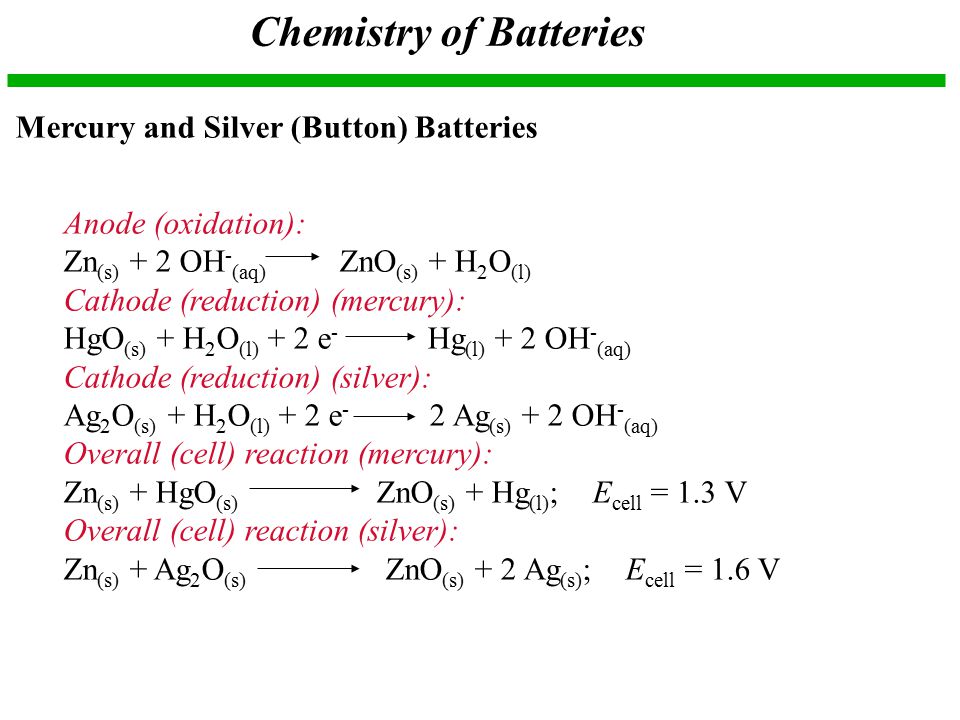
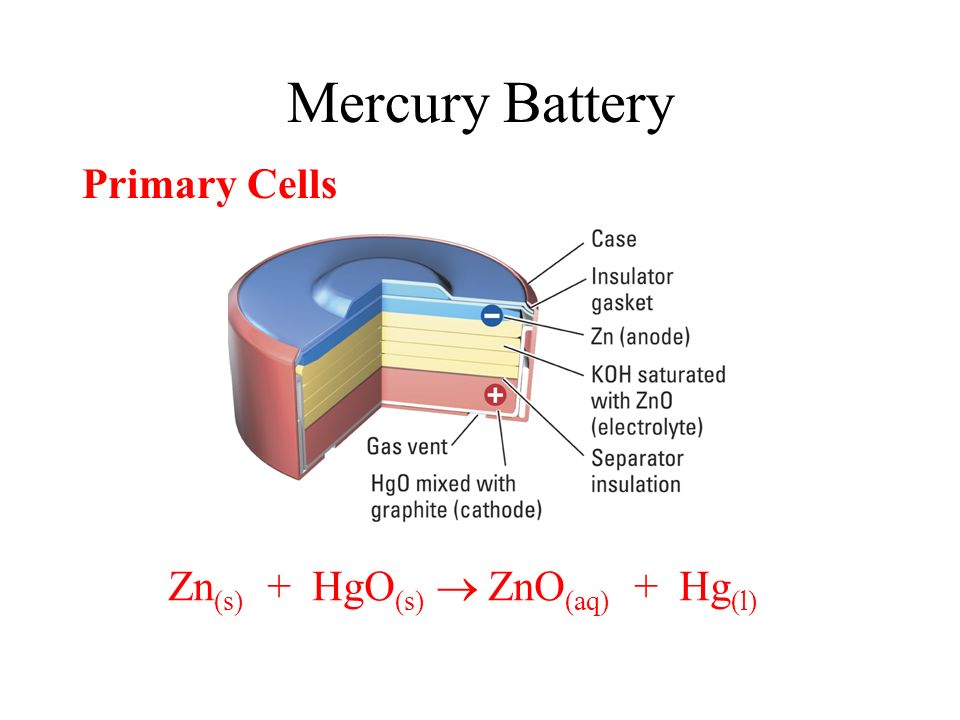
SOLVED:0.80 V For question (4), what does "X" represent? a) Tin b) Lead Cobalt Silver The net reaction of the mercury cell is Zn Hgo Hzo Zn(OH)2 Hg What substance is oxidized
a) Give anode and cathode reaction of mercury cell. (b) Calculate emf of the cell for the cell reaction at 25ºC for the cell : - Sarthaks eConnect | Largest Online Education Community

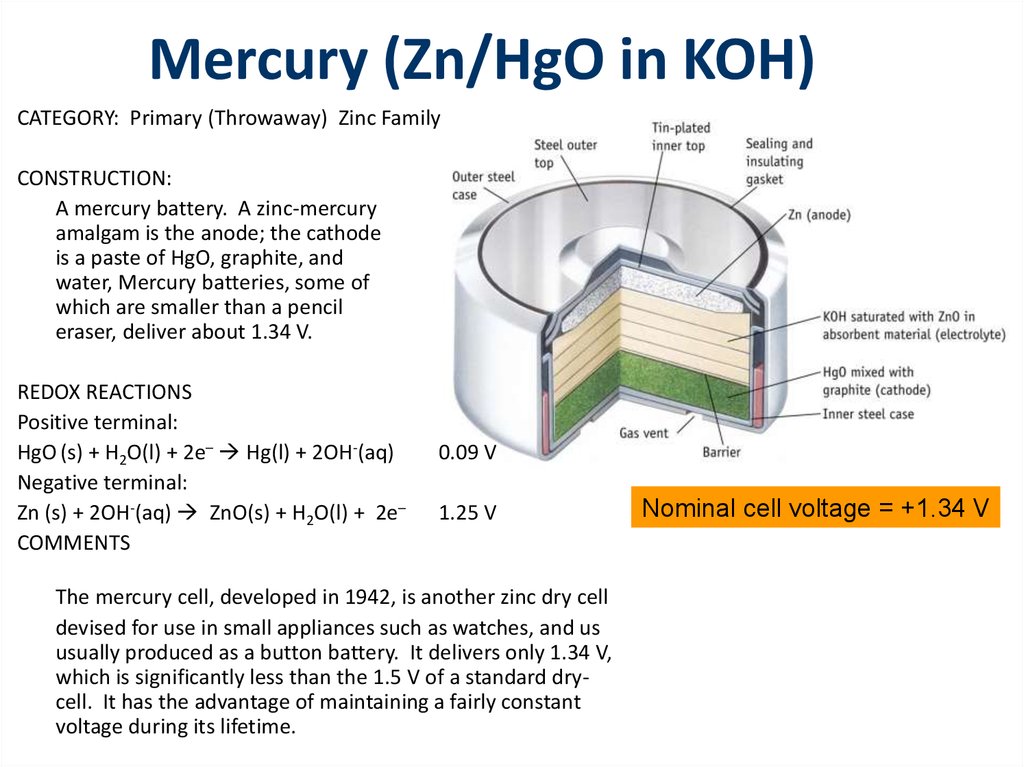

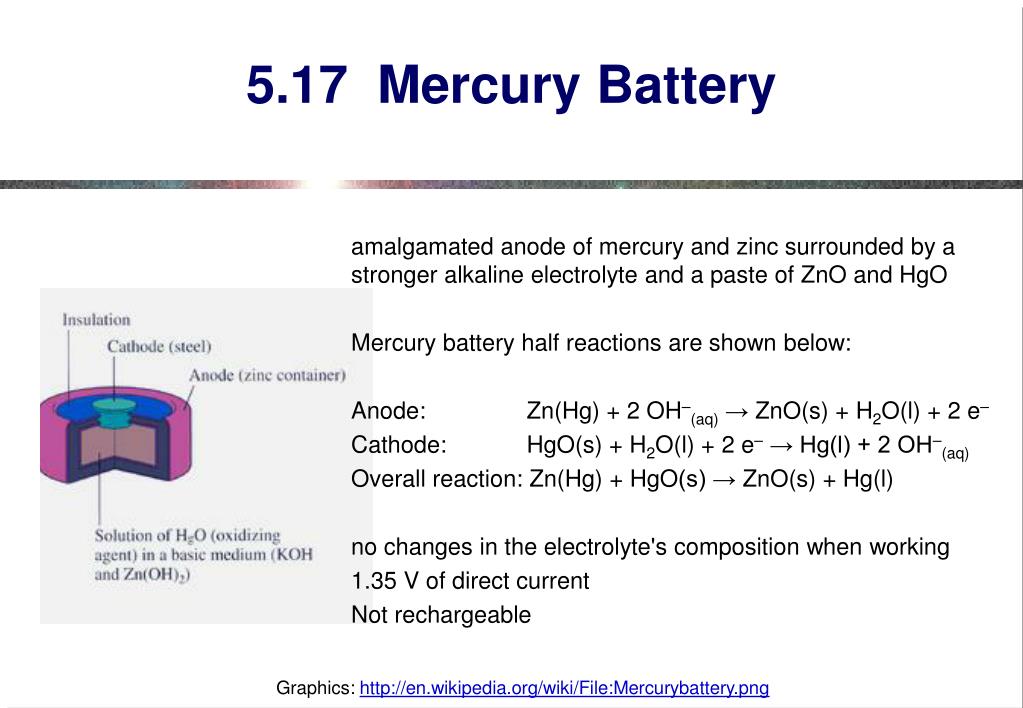





.jpg)