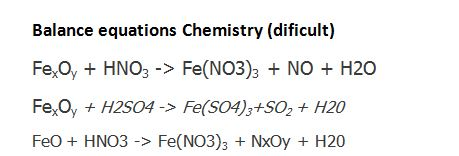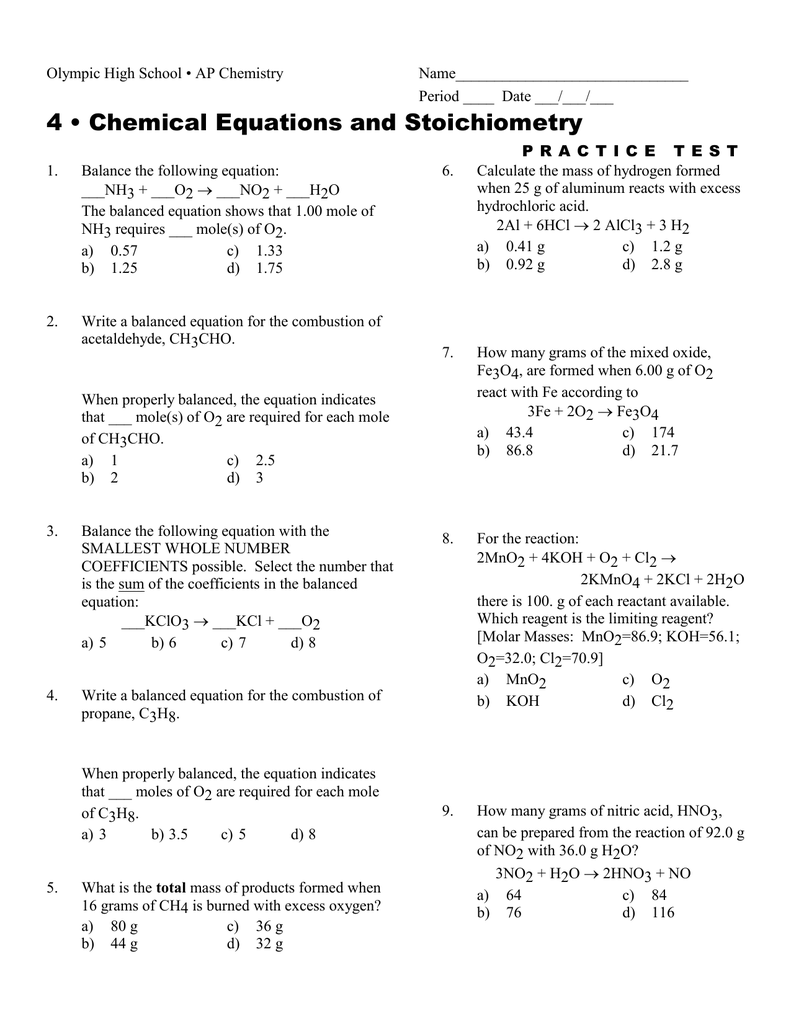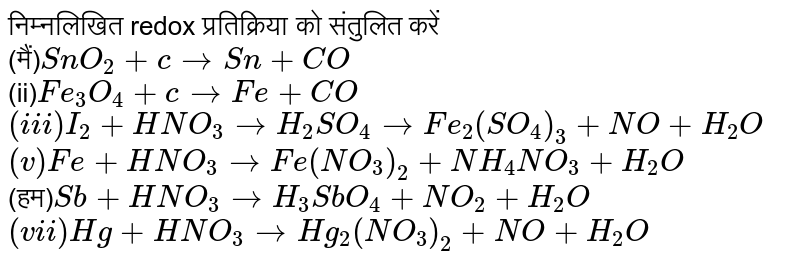
The ratio of coefficient of HNO_(3), Fe(NO_(3))_(2) and NH_(4)NO_(3) in the following redox reaction Fe + HNO_(3) rarr Fe (NO_(3))_(2) + NH_(4)NO_(3) + H_(2)O are respectively
Write balanced chemical equation for the following : 1. Reaction of nitric acid with sodium bicarbonate. - Sarthaks eConnect | Largest Online Education Community

Balance the following redox reaction FeS{O}_{4}+HN{O}_{3}+{H}_{2}S{O}_{4}xrightarrow{} {Fe}_{2}(S{O}_{4}{)}_{3}+NO+{H}_{2}O | Snapsolve

Balance the following chemical equation Fe(s) +H2O(g) = Fe3O4 + H2(g) MnO2 + HCL = MnCl2 + Cl2 + - Science - Chemical Reactions and Equations - 14408537 | Meritnation.com

The ratio of coefficient of HNO3,Fe (NO3)2 and NH4NO3 in the following redox equation, Fe + HNO3→Fe (NO3)2 + NH4NO3 + H2O in the balanced form will be?

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

Balance the given equation by oxidation number method - FeSO4 + HNO3 + H2SO4 = Fe(SO4)3 + NO + - Chemistry - Redox Reactions - 13629296 | Meritnation.com

The ratio of coefficients of HNO, Fe(NO3), and NH.NO, in the following redox reaction: Fe + HNO, → Fe(NO3)2 + NH.NO,+H,0 are, respectively, (a) 10:1:4 (c) 4:10:1 (b) 10:4:1 (d) 4:1:10
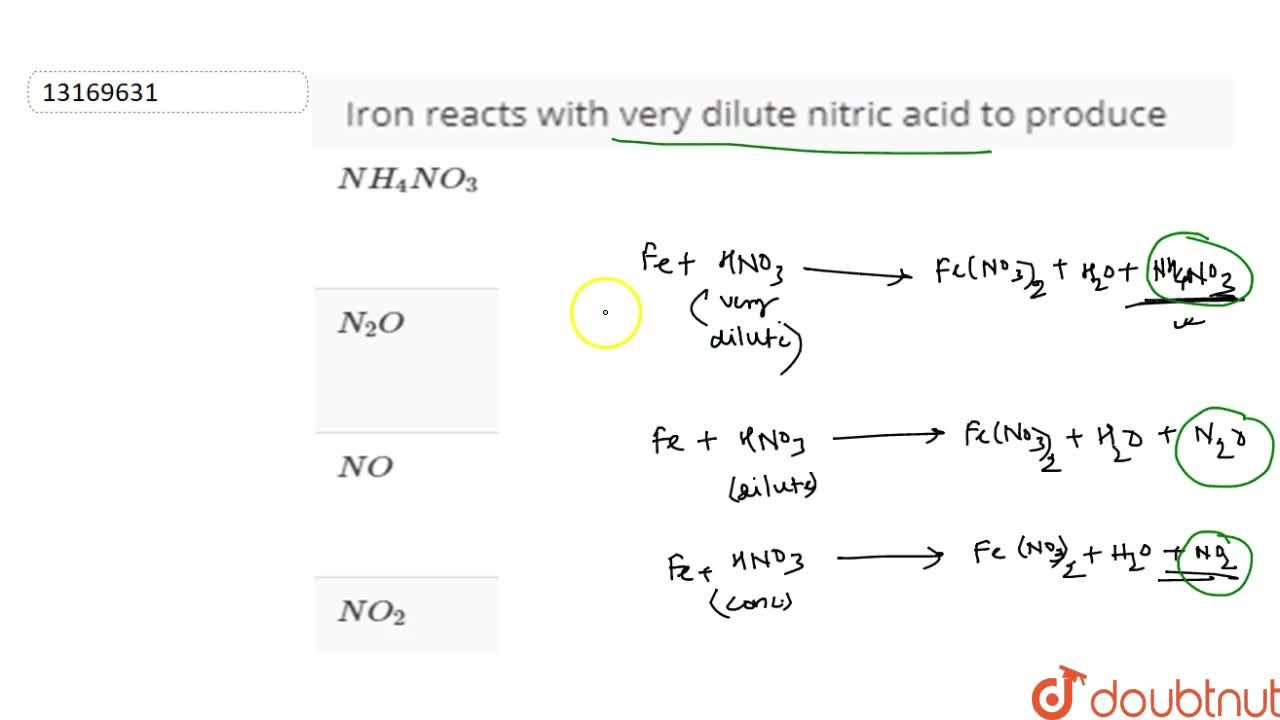
The reaction of iron with dilute HNO3 gives no reaction due to passivity or gives passivity only? Can anyone explain this to me because I can't understand this reaction in my chemistry

I HNO3 + Fe= II HCl + Fe = please answer immediately don't send link - Science - Materials Metals and Non-Metals - 13482205 | Meritnation.com